Can You Believe the History of Natural Soap Spans 4,000+ Years?
A Google search for 'soap recipe' returns 115,000,000 results. 115 Million. The wild thing is that soap recipes, to a one, date back to a single clay tablet, written in ancient Sumerian. Ancient barely begins to describe it. And despite many technological advancements, the art of crafting natural soap hasn't strayed far from its roots in ancient Mesopotamia over 4,000 years ago.
The Babylonian artisans who first started making soap (and recording their recipes) probably used ash from burnt hardwood trees, in combination with oil or animal fat. We have the receipts — the recipe is recorded in ancient Sumerian, on a clay tablet that survives to this day. (Pictured above)
And for the science geek in all of us, this recipe is not just the first soap recipe — it's also another turning point in human history; it's the earliest recorded chemical reaction.
In this article we'll explore the history of the soap making craft with a specific focus on lye (sodium hydroxide) — truly a misunderstood chemical. Indeed, without lye, soap as we know it today would not exist. So let's dive in!
A quick intro to the soap-making process
If we're going to understand lye's role in the history of soap making, we need to first understand a little bit about how soap is created.
In the earliest and most natural form, soap was made by mixing oil (triglycerides) together with a lye solution made from running boiling water over ashes (an akaline). Here's what that soap recipe might have looked like, using ashes from hard woods along with non-mineralized water.
Straining boiling water through ash would create an impure form of lye (sodium hydroxide). Combining the lye with oils creates a chemical reaction called sponification - which in turn produces fatty acid metal salts (the soap) and glycerol, a highly desirable by-product of the soap making process.

Indeed, glycerin is a moisturizing agent, and creates a layer on the skin that attracts water to the skin.
After this process is complete, there is no lye leftover. So while lye is an ingredient in the soap-making process, its presence is only temporary. The lye is entirely used up in the process and doesn't remain in the finished product.
Whew! Now that we've been through 'Chemistry 101', let's look at the history of soap making.
Ancient Alchemy: Lye's Role in Early Soap Making
A Glimpse into Ancient Egypt
Soap making is mentioned in the Ebers Papyrus, the oldest and most important compilation of herbal and medicinal knowledge in ancient Egypt, dating back to 1550 BC.

Along the fertile Nile River, the Egyptians used a soda ash substance called Trona, along with ashes from burnt crops.
Mixing these types of lye with valuable oils, they created early forms of soap. These artisans improved their techniques, combining these natural ingredients to make cleansing products that quickly became part of daily life, including as a preparation of wool for weaving.
Soap in the Roman Tradition
Moving to ancient Rome, we find another part of soap's history. The word "lye" comes from the proto-indo-european word 'leue' (to wash). The Romans, trading with germanic tribes, adopted a word for soap ('sapo') from the germanic 'sebum' (Tallow).
Germanic soap makers, as described by Pliny the Elder, a chronicler of life in the first century AD, were known for creating soaps less harsh than those made by the Romans.
The Islamic Golden Age
Recipes for soap-making are described during this period, circa 800 AD, by Muhammad ibn Zakariya al-Razi, an important philosopher and historian of the age.
A document from the 12th century illustrates the process of making soap. It mentions the key ingredient, alkali, which later became crucial to modern chemistry, derived from al-qaly or "ashes".
Soap making continued to develop so that by the 1200s, soap production had become a major cottage industry in the Middle East.
Medieval Europe transitions from animal fat to olive oil
In the 10th century, soap in Europe was still being made from animal fat, creating a soap with an effective cleaning agent but with an unpleasant odor. As recipes changed and olive oil was introduced, soap production moved to the Mediterranean region of Europe.
The Industrial Revolution and Mass Production
The industrial revolution marked a profound transformation in the soap-making world. Mass production methods and the use of chemical additives were introduced. While these changes significantly increased the accessibility of soap to the masses, they also marked a departure from the earlier traditions of using natural, simpler ingredients.
The collapse of small soap manufacturers
England introduced a suite of taxes and regulations on soap making from the late 1600s to the mid 1800s. Soap became a luxury. By law, soap boilers had to produce an industrial-sized lot of soap in each production round, leading to the craft of soap making moving beyond the reach of small manufacturers.
Industrial soap production started in the mid-1800s, coinciding with the lifting of the tax on soap, in 1853.
Mass-produced soap, although practical, often came at the expense of the artisanal and traditional aspects that once defined soap making. Harsh additives and artifical fragrences in soap contribute to skin irritation and increase the exposure of our bodies to harmful ingredients.
A Modern-Day Renaissance
Today, the world of soap making is experiencing a revival of the ancient craft.
There's a renewed interest in artisanal methods and a return to the use of natural ingredients. This resurgence speaks to our enduring connection with the past and our growing appreciation for the artistry that has shaped the soap we use in our daily lives. It is a testament to the timelessness of this craft and its unwavering significance throughout the ages.
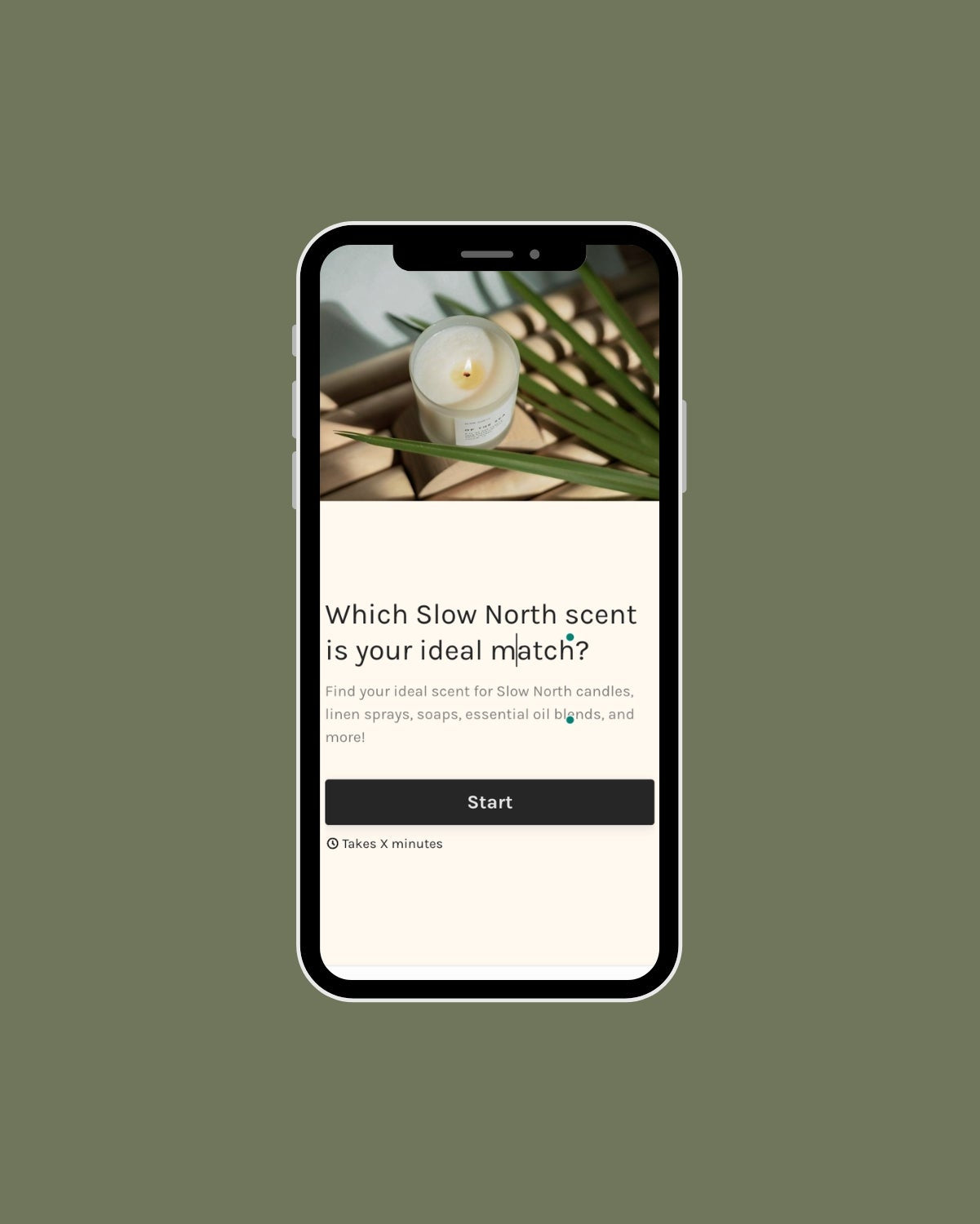
If you're looking for natural bar soaps, Slow North creates small-batch soap using 100% essential oils and organic ingredients.
Chemistry: The Magic at the Heart of Soap Making
The Saponification Process with Lye
The process of saponification includes breaking ester bonds in triglycerides, the molecules in fats and oils. This breakage creates glycerol and soap molecules, which are mainly sodium or potassium salts of fatty acids. These soap molecules have special properties that let them bind effectively to both water and oil, making them exceptional cleansing agents.
More About Lye
Lye, often called the double-edged tool of soap making, has an interesting two-sided nature. Lye, also known as sodium hydroxide for solid soap or potassium hydroxide for liquid soap, is at the center of soap making. Lye used in soap-making is a strong alkali substance, which means it can be harmful if not handled carefully. However, it's this very chemical makeup that makes it a necessary part of the saponification process.

Safely Using Lye
The balance between the risks and benefits of lye is the key here. For ages, soap makers have been careful when using it. If allowed to finish the saponification process, lye doesn't remain in the finished product, and isn't technically an ingredient in the final soap product. Soap makers use a deficit of lye in their recipes to make sure that all the lye is transformed in each batch so that none remains at the end of the soap-making process.
Modern soap makers know how important safety is. They wear protective gear, make sure there's good airflow, and handle lye very carefully. This leads to a safe and gentle bar of soap, made by carefully working with this unusual substance.
Final Thoughts
From its origins in ancient civilizations to its important role in modern soap making, lye is a symbol of change and renewal, linking us to traditions that have enriched our lives for ages. It's a reminder of the enduring importance of this simple yet remarkable substance that's been key in giving us one of life's essential daily luxuries – soap.
FAQs: Questions About Lye and Soap-Making
Lye remains a mysterious ingredient in the world of soap making, sparking curiosity and questions. In this section, we'll provide answers to some of the most frequently asked questions about lye and its role in crafting various types of soap.
Is lye safe for the skin?
Unless you are making soap, you don't need to worry about lye. During the soap-making (saponification) process, all the lye is 'used up' and doesn't remain in the soap. So any soap you buy on the shelves are safe for your skin.
If, however, you are working with lye during the soap making process, take care - as lye is not safe for the skin.
Sodium hydroxide (lye) is a strong base (the opposite of an acid). When soap is made, the lye reacts with oils and fats, creating a type of salt that is the soap we know and love.
Lye can react with the skin and cause chemical burns, which is why soap making always includes goggles and the proper type of gloves for hand protection.
In soap making, the lye reacts completely with the oils and fats, meaning that there is actually no lye in the soap we buy off the shelves (and at the stands of maker's markets).
So yes, when used in the proper proportions and allowed to complete the saponification process, the lye in soap is entirely safe for the skin. In fact, lye is a necessary component for transforming oils and fats into soap.
What safety measures should be taken when working with lye?
Safety measures when working with lye include wearing protective gear, such as gloves and goggles, ensuring proper ventilation, and carefully measuring and mixing lye. It's essential to follow a reliable soap-making recipe and instructions to ensure safe handling.
Can you make lye from scratch?
While it is possible to create lye from wood ashes through a labor-intensive process, most modern soap makers prefer to use commercially available lye. Commercially produced lye is more consistent and easier to work with.
Is lye used in all types of soap?
Lye is a fundamental ingredient in the soap-making process, whether it's for bar soaps, liquid soaps, or even specialty soaps like glycerin soaps. While there are different types of lye, such as sodium hydroxide for solid soaps and potassium hydroxide for liquid soaps, they all play a crucial role in soap making.